

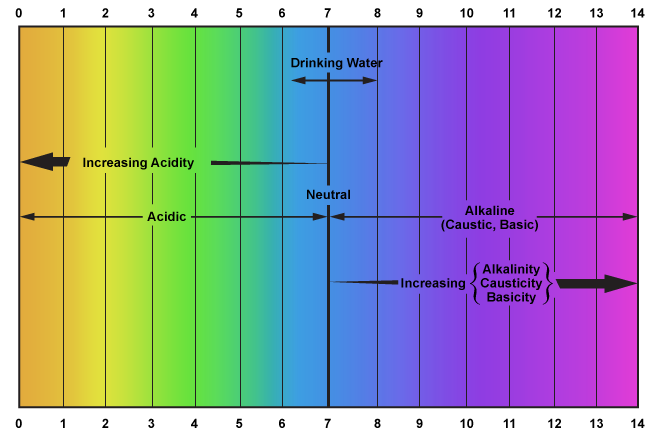
The pH of a solution expresses its relative acidity or alkalinity on a scale of 0 to 14. The pH expresses the concentration of the hydrogen ion (H+). Pure distilled water has a pH value of 7 and is regarded as neutral (neither acidic nor alkaline). The pH values decreasing from 7 to 0 indicate increasing acidity, and pH values increasing from 7 to 14 indicate increasing alkalinity.
Solutions contain chemical species called ions. An ion is an electrically charged particle obtained from an atom or a chemically bonded group of atoms by adding or removing electrons. Electrons carry a negative charge. Because of this, ions with extra electrons carry a negative charge and ions missing electrons carry a positive charge. Two important species in virtually all solutions are the hydrogen ion (H+) and the hydroxide ion (OH-). The H+ and OH- ions combine to form water as shown in the following formula:
H+ + OH-→H2O
Acid solutions have an excess of uncombined H+ ions, and alkaline solutions have an excess of uncombined OH- ions. To acidify a solution, H+ ions are added—from sulfuric acid (H2SO4), for example. To increase the pH of a solution, OH- ions are added—from sodium hydroxide (NaOH), for example—to combine with the excess H+ ions.
Each unit on the pH scale represents a ten-fold change in concentration. For example, a solution with a pH of 1 is 10 times more acidic than a solution with a pH of 2.
Refer to the table for the pH values of some common solutions and materials.